What is the maximum number of emission lines when the excited electron Hydrogen series bohr spectrum atom chemistry line energy model theory levels transitions wavelength spectral emission lines spectra atomic diagram different What is the maximum number of emission lines when the excited electron
Absorption and Emission Lines
Emission spectra of hydrogen & calculation of number of emission lines Hydrogen spectrum series atom lyman lines atomic chemistry structure which balmer class paschen number pfund brackett classnotes Emission electron atomic hydrogen bohr spectra emitted atoms photon balmer orbits wavelength spectroscopy electrons orbit spectral libretexts quantized cognitive theoretic
Emission absorption spectrum wavelength continuum s7 flux superimposed cosmos swin
Spectral linesHydrogen spectrum emission atomic atom rydberg formula series frequency spectral equation scale atoms wavelength bohr chemistry terms libretexts theory pfund What is the maximum no.of emission lines when the excited electron of aSpectrum atomic lines gas bright atoms spectra wavelengths molecules hydrogen characteristic different emission line spectral color light leads colors types.
Lines maximum emission number when excited atom electron drops ground state questionKnowledge sea: atomic spectrum Emission lineHydrogen spectrum.

Emission lines hydrogen spectra
Emission brainly electron atomAbsorption and emission lines Emission spectrum arroz atom photons emitted discrete fato q132 cozinhar enemEmission spectrum light state do energy electron hydrogen electrons absorption physics question objects does levels transition quantum chemistry chemical visible.
Hydrogen's atomic emission spectrumLines spectral calculate bohr model Bohr model to calculate emitted spectral linesChemistry theory and revision.

7.3: atomic spectroscopy and the bohr model
Lines spectral number outline help answerEmission line Answered: hydrogen atoms are excited by a laser…Emission drops atom electron shaalaa hence.
Lines spectrum emission absorption spectral spectra continuous hydrogen atomic line light gas dark chemistry fraunhofer show chem figure theory emissionsAbsorption emission hydrogen atom electron h2 .


Hydrogen spectrum | Chemistry, Class 11, Structure Of Atom

Spectral Lines

Emission Spectra of Hydrogen & Calculation of Number of Emission Lines

7.3: Atomic Spectroscopy and The Bohr Model - Chemistry LibreTexts

Absorption and Emission Lines

Hydrogen's Atomic Emission Spectrum - Chemistry LibreTexts

Chemistry - Electron Emission Spectrum
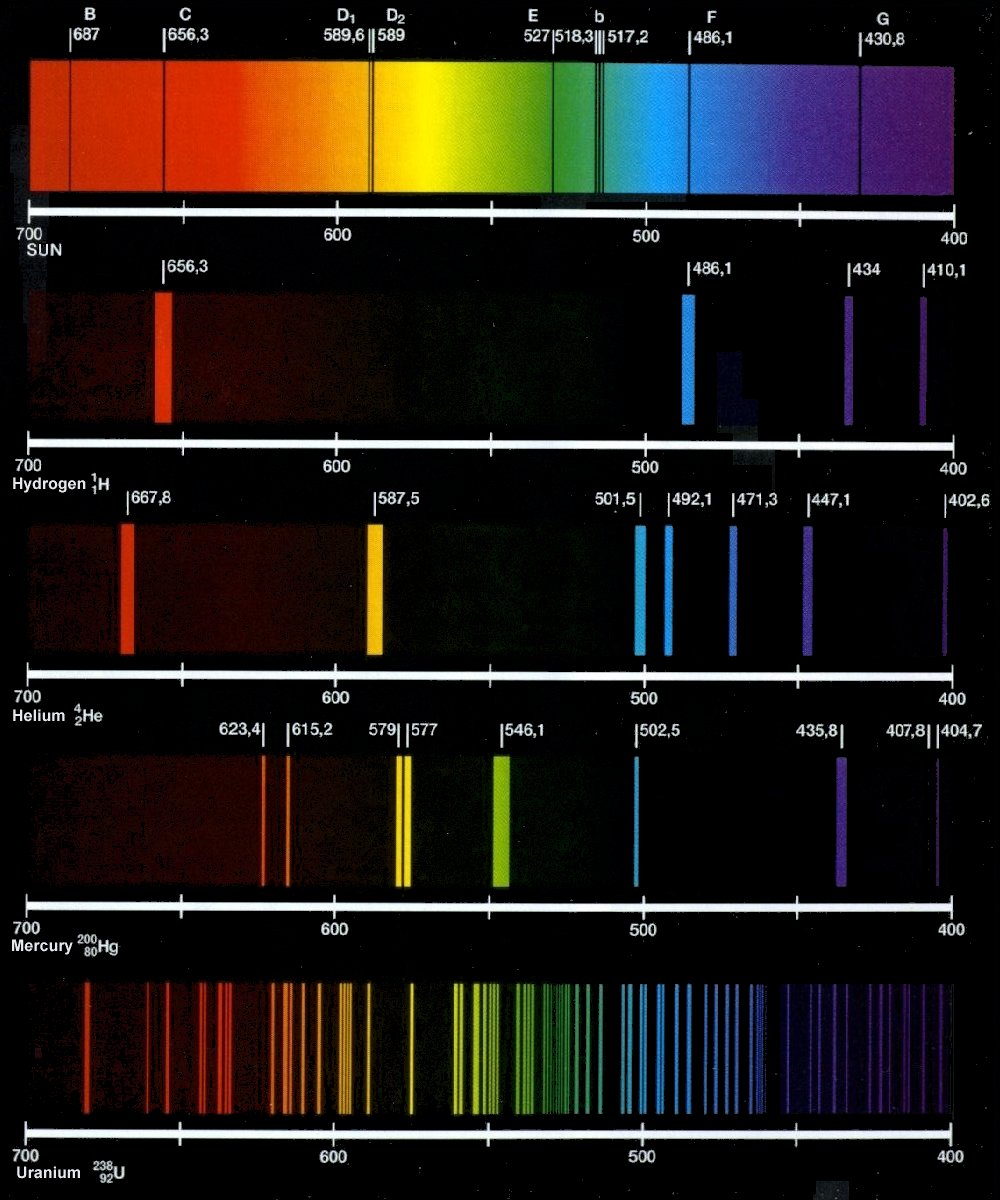
knowledge sea: ATOMIC SPECTRUM

What is the maximum no.of emission lines when the excited electron of a
